Steroids biological role. The role of steroid hormones in the body. More complex types of testosterone
Steroids do not dissolve in water, but are perfectly soluble in all fatty solvents. If the fat is saponified, then the steroids remain in the unsaponifiable fraction, from which they can be isolated in pure form by fractional crystallization from alcohol solutions.
Steroids play an important role in the composition of protoplasm, forming complex complexes with proteins involved in the construction of intracellular membranes.
The content of steroids in yeast is especially high, which is used for the industrial isolation of ergosterol and the subsequent production of vitamins of group D from it. Yeast contains over 2% of steroids per dry matter. Their wheat grain contains from 0.03 to 0.07%; in corn grain - from 1 to 1.3%.
Significant amounts of steroids (ergosterol) are contained in the mycelium of mold fungi.
Plant Steroids
Steroids- derivatives of. Steroids include sterols and their derivatives, some sapogenins, which are part of saponins, cardiac glycosides and aglycones of glycoalkaloids, and a number of hormones of animal origin.
Steroids do not dissolve in water but perfectly soluble in all fatty solvents. Therefore, when extracting any product of plant origin with sulfuric ether or other fatty solvent, sterols also pass into the extract in addition to fats and phosphatides. If the fat is saponified, then the sterols remain in the so-called unsaponifiable fraction, from which they can be isolated in pure form by fractional crystallization from alcohol solutions.
Steroids play an important role in the composition of protoplasm, forming complex complexes with proteins involved in the construction of intracellular membranes; the value of the latter is very significant in the regulation of metabolism in the cell.
Representatives of steroids
A characteristic representative of the sterol group is ergosterol C28H43OH. It is found in yeast, ergot horns, molds, and wheat grains. As A. Windaus showed, from ergosteral, when it is irradiated with ultraviolet rays, vitamins of group D are formed.
A number of sterols have been isolated from various plant products. Thus, a sterol having the empirical formula C27H45OH has been isolated from corn oil and wheat germ oil. The oil obtained from wheat endosperm contains two sterols - one with the same empirical formula C27H44OH and the other (its hydrogenated derivative) - dihydrosterol, corresponding to the empirical formula C27H47OH. Sterols with the empirical formula C30H49OH have also been isolated from wheat and rice germs. Individual sterols differ from each other in the number of double bonds they contain and in the structure of the side chain. For example, sitosterols С29Н49ОН - a group of sterols very common in plants - unlike ergosterol, contain only one double bond, and in their side chain one methyl group is replaced by an ethyl one.
Stigmasterol C29H47OH, contained in soybean oil, and spinasterols isolated from spinach and cabbage leaves differ from sitosterol by the presence of two double bonds in them. Especially high is the content of sterols in yeast, which is used for the industrial isolation of ergosterol and the subsequent production of vitamins of group D from it. Yeast contains over 2% of sterols on a dry matter basis. Their wheat grain contains from 0.03 to 0.07%; in corn grain, which is characterized by a high fat content, from 1.0 to 1.3%.
Significant amounts of sterols, in particular ergosterol, are contained in the mycelium of mold fungi, which remains as a waste product in the production of antibiotics and citric acid. In bacteria, sterols are either not contained at all, or are contained in a very small amount (from 0.0004 to 0.01% per dry matter). The content of sterols in the leaves is low - about 0.05-0.18% per dry matter. Of great interest is the presence in higher plants of insect hormones: juvenile hormone and molting hormones - ecdysones. To date, about 40 different ecdysones with high biological activity have been isolated from many plants. The content of ecdysones in plants reaches 2%. The starting material in the biosynthesis of sterols, as well as in the biosynthesis of all terpenoids, is the acetyl residue CH3CO-.
Steroids include a whole group of hormones that are produced in the gonads and adrenal glands. Sex hormones are derivatives of cholesterol, which is considered to be so harmful to our body. But without it, there would be no person, since it is the main raw material for hormones, and procreation is simply impossible without them.
| Article structure: |
This is such a dual function of cholesterol, which, on the one hand, destroys many people, clogging blood vessels and causing the development of very serious diseases, and on the other hand, it makes it possible for life to arise, albeit indirectly. But today is not about cholesterol, but about steroid hormones, their differences and effects on the body.
Classification of steroid hormones.
In the sex glands are formed:
- androgens;
- estrogens;
- progestins.
The adrenal glands synthesize:
- glucocorticoids;
- mineralcorticoids.
All these hormones are called steroids, only the difference between them is significant, each of them is responsible for its own function. In the gonads, thanks to cholesterol, the main male hormone, testosterone, and the female hormone, estradiol, are synthesized. Although their functions are radically different from each other, it is these hormones that are responsible for male and female characteristics, respectively, which is the difference between the sexes. But their structure is very similar, and the reason for this is the common derivative from which they appeared, that is, cholesterol.
The fact that testosterone is a steroid, as it is called in medical terminology, is known to many, but other biochemical compounds are included in this group. For example, corticosteroids, glucocorticosteroids and estrogens, and if the main task of testosterone is the development of male characteristics, from the timbre of the voice to a certain type of figure, and estrogens for female characteristics, then gluco- and corticosteroids have a completely different meaning and are not involved in the body structure of one sex or another. This is all to the fact that many people associate the concept of a steroid with muscle growth, but only testosterone and its derivatives have this function, and not all hormones in a row, which are also called steroids.
Androgenic and anabolic properties.
So, for the possibility of additional growth muscle tissue, testosterone is used in sports. Drugs called steroids may have a different name than testosterone, but they will always be based on this male hormone, and other compounds will be added to it to enhance this or that effect. Testosterone has two main groups of functions:
- androgenic;
- anabolic.
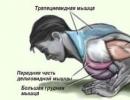
For athletes, it is the anabolic effect of a synthetic hormone that is of particular importance. The anabolic side is responsible for the structure of the musculoskeletal system according to the male type, that is, a narrow pelvis, broad shoulders and ratio muscle mass, to a general physique that greatly exceeds the female musculature. It is for this very muscle growth, and also for strength and endurance, which are also characteristic of the anabolic action of testosterone, that athletes around the world are chasing. The androgenic side is responsible for secondary male characteristics, such as the growth of body hair, a certain timbre of voice, the formation of genital organs, and so on.
When they began to produce synthetic steroids for use in sports, that is, the hormone testosterone in the laboratory, the main idea was to leave only anabolic properties. The idea, of course, is excellent, but, unfortunately, completely impracticable. As it turned out in practice, in testosterone there are no separate cells responsible for androgenic activity and separately for anabolic activity. They are a single whole, it was impossible to separate them. And then these hormones were called androgenic-anabolic steroids, which activate both one and the other functions. Since it is not possible to completely separate these functions, it is not entirely correct to call drugs anabolic steroids, because the androgenic side is also inherent in them. But over time, pharmacology has managed to recreate drugs with an anabolic or, conversely, androgenic advantage. For example, drugs such as: Winstrol, Deca-Durabolin or Anadrol have a pronounced anabolic effect on the body, which on average exceeds androgenic by 250%. It is for such steroids that bodybuilders are chasing to increase muscle mass, since they do not need androgenic activity at all.
How testosterone affects muscles.
The fact that it is testosterone derivatives that are responsible for the process of muscle growth has been sorted out. But how does it make muscle tissue grow, and not, for example, adipose tissue? It's all about the receptors that are sensitive to this hormone. So, in every cell of the body, there are receptors that react to one or another element moving along with the flow of blood and lymph. When a compound suits it, the receptor absorbs it; if not, it does not react to it. In the muscle mass there are receptors that respond to testosterone and in men there are more of them in the upper body than in women, this also determines the different structure of the figure. The higher the testosterone level, the more receptors are formed and the large quantity they trap the hormone. In fact, this is a circular reaction, more testosterone enters the body, more receptors become. This is why athletes who systematically inject themselves with testosterone manage to grow to such an impressive size.
Summing up, I would like to note that androgenic steroids not only improve protein synthesis in muscle tissue, but also do not allow catabolic hormones to act. The latter act destructively on muscle mass, in bodybuilding there is such a thing as protein catabolism in muscles, that is, the destruction of protein to the state of amino acids and, accordingly, a decrease in muscle mass. The task set for steroids is to start anabolic processes and suppress catabolism, which, however, they cope with quite successfully. These are the interesting properties that androgenic anabolic steroid that have become so popular in different circles of athletes. As in any other chemically synthesized drugs, steroids bring both benefit and harm to the body, which effect will be more pronounced depends on many factors. Whether or not to take these drugs is up to you.
Steroids have different origins: natural (animal, plant) and artificial. They are used in medicine and sports.
The classification of steroids is based on their structure and origin:- sterols are steroid alcohols, which include cholesterol, ergosterol, stigmasterol;
- steroid hormones;
- bile acids are formed in the liver during the processing of cholesterol, they provide the digestion of lipids;
- alkaloids, cardiac glycosides, saponins, etc.
- increase appetite;
- accelerate the regeneration of the body;
- contribute to an increase in body weight, due to which there is a decrease in the level of body fat in relation to total mass body;
- calcium and phosphorus accumulate better in bones and teeth;
- increase efficiency and endurance;
- improves the filling of blood vessels and brain activity;
- the feeling of fear decreases, self-confidence and communication skills increase.
- the occurrence of acne;
- increased irritability and frequent mood swings, depression;
- hypertension;
- atherosclerosis due to increased cholesterol levels;
- negative effect on the liver, myocardium;
- impotence, reduced sperm production, infertility, testicular atrophy;
- accumulation of excess fluid;
- breast enlargement in men, etc.
Side effects appear if there is a violation of the dosage of the drug. If you do not allow abuse, then you can reverse the negative consequences. Before starting the course, consult your doctor and carefully study the instructions.
Send your good work in the knowledge base is simple. Use the form below
Students, graduate students, young scientists who use the knowledge base in their studies and work will be very grateful to you.
Posted on http://www.allbest.ru/
RSEonPVC"WESTERN- KAZAKHSTANstatemedicaluniversitynameMaratospanova"MOHRK
Faculty: Pharmacy
Department: Chemical disciplines with a course of pharmaceutical disciplines
IndependentJobstudent
Discipline: General methods of research and analysis of drugs
Subject: The biochemical role of steroids in the body as a prerequisite for obtaining medicinal substances.
Performed: Tulegenova Nurslu
Aktobe 2015
Introduction
There are lipids that are not hydrolyzed to release fatty acids. These lipids include steroids.
Steroids are compounds widely distributed in nature. They are often found in association with fats. They can be separated from fat by saponification (they fall into the unsaponifiable fraction). All steroids in their structure have a core formed by hydrogenated phenanthrene (rings A, B and C) and cyclopentane (ring D):
Steroids- substances of animal or, more rarely, plant origin, with high biological activity. Steroids are formed in nature from isoprenoid precursors. A feature of the structure of steroids is the presence of a condensed tetracyclic system of gonan. The gonan core in steroids can be saturated or partially unsaturated, contain alkyl and some hydroxyl, carbonyl or carboxyl functional groups.
Steroids- complex organic compounds, derivatives of substituted.
Steroids are quite widely represented in wildlife.
A feature of steroids is the presence of hydroxyl or keto groups in the third position of the cycle. Depending on the structure of the A- and B-cycles, the location and nature of the substituents in the molecule and the nature of the side chain, as well as the properties and biological action, steroids are divided into sterols, vitaminsgroupsD, bileacids, alcohols,steroidsaponins,alkaloids, hormones, biologicallyactiveconnectionsplants,antibiotics(cephalosporin).
Most steroids in the body perform an important regulatory function. Synthesis of steroids is carried out in the body of plants and higher animals and humans. The precursor of animal and plant steroids is squalene, which is converted to steroids with the participation of triterpenoid alcohols or cycloartenol (in plants).
Steroids do not dissolve in water, but are perfectly soluble in all fatty solvents. If the fat is saponified, then the steroids remain in the unsaponifiable fraction, from which they can be isolated in pure form by fractional crystallization from alcohol solutions.
Steroids play an important role in the composition of protoplasm, forming complex complexes with proteins involved in the construction of intracellular membranes.
The content of steroids in yeast is especially high, which is used for the industrial isolation of ergosterol and the subsequent production of vitamins of group D from it. Yeast contains over 2% of steroids per dry matter. Their wheat grain contains from 0.03 to 0.07%; in corn grain - from 1 to 1.3%.
Significant amounts of steroids (ergosterol) are contained in the mycelium of mold fungi.
SteroidsNotdissolveVwater but perfectly soluble in all fatty solvents. Therefore, when extracting any product of plant origin with sulfuric ether or other fatty solvent, sterols also pass into the extract in addition to fats and phosphatides. If the fat is saponified, then the sterols remain in the so-called unsaponifiable fraction, from which they can be isolated in pure form by fractional crystallization from alcohol solutions.
SteroidsplayimportantroleVcompositionprotoplasm, forming complex complexes with proteins involved in the construction of intracellular membranes; the value of the latter is very significant in the regulation of metabolism in the cell.
A characteristic representative of the sterol group is ergosterol C28H43OH. It is found in yeast, ergot horns, molds, and wheat grains. As A. Windaus showed, from ergosteral, when it is irradiated with ultraviolet rays, vitamins of group D are formed.
A number of sterols have been isolated from various plant products. Thus, a sterol having the empirical formula C27H45OH has been isolated from corn oil and wheat germ oil. The oil obtained from wheat endosperm contains two sterols - one with the same empirical formula C27H44OH and the other (its hydrogenated derivative) - dihydrosterol, corresponding to the empirical formula C27H47OH. Sterols with the empirical formula C30H49OH have also been isolated from wheat and rice germs. Individual sterols differ from each other in the number of double bonds they contain and in the structure of the side chain. For example, sitosterols С29Н49ОН - a group of sterols very common in plants - unlike ergosterol, contain only one double bond, and in their side chain one methyl group is replaced by an ethyl one.
StigmasterolC29H47OH, contained in soybean oil, and spinasterols isolated from spinach and cabbage leaves differ from sitosterol by the presence of two double bonds in them. Especially high is the content of sterols in yeast, which is used for the industrial isolation of ergosterol and the subsequent production of vitamins of group D from it. Yeast contains over 2% of sterols on a dry matter basis. Their wheat grain contains from 0.03 to 0.07%; in corn grain, which is characterized by a high fat content, from 1.0 to 1.3%.
Significant amounts of sterols, in particular ergosterol, are contained in the mycelium of mold fungi, which remains as a waste product in the production of antibiotics and citric acid. In bacteria, sterols are either not contained at all, or are contained in a very small amount (from 0.0004 to 0.01% per dry matter). The content of sterols in the leaves is low - about 0.05-0.18% per dry matter. Of great interest is the presence in higher plants of insect hormones: juvenile hormone and molting hormones - ecdysones. To date, about 40 different ecdysones with high biological activity have been isolated from many plants. The content of ecdysones in plants reaches 2%. The starting material in the biosynthesis of sterols, as well as in the biosynthesis of all terpenoids, is the acetyl residue CH3CO-.
The three most important groups of steroids are sterols, bile acids and steroid hormones. In addition, steroids include plant compounds with valuable pharmacological properties: steroidal alkaloids, digitalis glycosides (cardiac glycosides) and steroidal saponins.
Steroid alcohols are called sterols. All sterols contain a β-hydroxyl group at C-3 and one or more double bonds in the B ring and side chain. Sterol molecules lack carboxyl and carbonyl groups.
Cholesterol is the most important sterol in animals. Plants and microorganisms contain many related compounds, such as ergosterol, β-sitosterol, stigmasterol.
Cholesterol is present in all animal tissues, especially in nervous tissues. It is an essential component of cell membranes, where it regulates their fluidity (see p. 219). Spare and transport forms of cholesterol are its esters with fatty acids. Along with other lipids, cholesterol and its esters are present in the lipoprotein complexes of blood plasma (see p. 273). Cholesterol is found in bile and many gallstones. Biosynthesis, metabolism and transport of cholesterol are discussed elsewhere (see pp. 175,305).
Cholesterol metabolism disorders play an important role in the development of atherosclerosis, a disease associated with the deposition of cholesterol (plaques) on the walls of blood vessels (calcification) due to increased levels of olesterol in the blood. To prevent atherosclerosis, it is important that the diet is dominated by plant products, which are characterized by a low cholesterol content. Against, food products animal origin contain a lot of cholesterol, especially egg yolk, meat, liver, brains.
Bile acids
Bile acids are formed from cholesterol in the liver (see p. 307). By chemical structure, these compounds are close to cholesterol. Bile acids are characterized by the presence of a shortened branched side chain with a carboxyl group at the end. There is no double bond in the B ring, and the A and B rings are joined in the cis position (see p. 61). The steroid core contains from one to three β-hydroxyl groups in positions 3, 7, and 12.
Bile acids ensure the solubility of cholesterol in bile and aid in the digestion of lipids (see p. 265). In the liver, primary bile acids are first formed - cholic and chenodeoxycholic (anthropodeoxycholic). Dehydroxylation of these compounds at C-7 by the intestinal microflora leads to the formation of secondary bile acids - lithocholic and deoxycholic.
Steroid hormones
The biosynthesis of steroid hormones - a process that is not so noticeable in quantitative terms - is, however, of great physiological importance. Steroids form a group of lipophilic signaling substances that regulate metabolism, growth, and reproductive functions of the body.
There are six steroid hormones in the human body: progesterone, cortisol, aldosterone, testosterone, estradiol and calcitriol (obsolete name calciferol). With the exception of calcitriol, these compounds have a very short two-carbon side chain or none at all. Most compounds of this group are characterized by the presence of an oxo group at C-3 and a conjugated C-4 / C-5 double bond in ring A. Differences are observed in the structure of rings C and D. In estradiol, ring A is aromatic and, therefore, the hydroxyl group has the properties of a phenolic OH groups. Calcitriol differs from vertebrate hormones, but is also built on the basis of cholesterol. Due to the light-dependent ring-opening reaction B, calcitriol forms the so-called “secosteroid” (ring-opened steroid).
Ecdysone, an insect steroid hormone, is an evolutionarily older formusteroid. Steroid hormones that perform a signaling function are also found in plants.
Factors that determine the effectiveness of taking steroids
steroid protoplasm hormone acid
Let's try to consider in detail what determines the effectiveness of steroids on the human body. All factors affecting efficiency can be divided into two large groups- external and internal.
Internal factors include the genetically predetermined density of receptors, their distribution in body tissues. The reaction of the body to steroids, as well as the degree of manifestation of side effects, largely depends on these factors. So, if the body has a high density of receptors in muscle tissue and a relatively low number of them in other tissues and organs, then in this case the anabolic response even to medium doses of drugs will be well pronounced, and the manifestation of side effects will be minimal, or they will not appear at all. . Otherwise, the result will be the opposite. Unfortunately, there are currently no available methods to determine the density and distribution of receptors in the body and to predict the response to steroid use.
Similarly, the molecular structure of the receptor itself may different people differ. This determines the fact that people have different reactions to the same drugs and their combinations.
The ratio plays a significant role. various types fibers in the muscles. The higher the percentage of fast twitch fibers and intermediate fibers, the better the response to steroids, of course, in terms of increasing muscle mass and strength.
The innate activity of liver microsomal enzymes and the activity of hepatic aromatase are also important. The higher the activity of these enzymes, the faster the rate of metabolism (destruction) of steroids in the body in the first case, and more of them undergo aromatization into estrogen in the second. All this reduces the effectiveness of the drugs introduced into the body and requires the introduction of large doses, or the adoption of special measures to combat aromatization, or antiestrogen therapy.
The next in turn, but not in importance, is the innate reserve of the cardiovascular system and the genetically determined strength of the ligamentous-articular apparatus and the framework of muscle tissue formed by connective tissue fibers. These factors make it possible to significantly increase the intensity strength training, as well as a long time to train intensively without injury, even when using high dosages of steroids. A well-developed capillary network in the muscles allows you to effectively deliver all the necessary nutrients, hormones, enzymes to the cells, as well as quickly remove metabolic products from the muscles.
And another very important factor. This functional state gastrointestinal tract. The more nutrients per unit of time the digestive system can process and assimilate, the higher the potential of a strength athlete. Internal factors are genetically predetermined and practically do not depend on external influences.
Conclusion
Steroids include numerous and extremely important classes of natural physiologically active compounds - sex hormones and hormones of the adrenal cortex, sterols, bile acids, sapogenins, cardiac aglycones, numerous alkaloids, etc. The prevalence of steroid compounds in both the animal and plant kingdoms and the extremely important role of steroid hormones in the regulation of life processes have led to a wide scope of scientific research during which important practical results have been obtained. Now in many countries industrial production of steroid hormones with products worth hundreds of millions has been established. Thanks to steroid hormones and especially cortisone, it became possible to cure such serious diseases as rheumatoid arthritis.
Literature
1.A.P. Arzamastsev. Pharmaceutical Chemistry: tutorial, 3rd ed., rev. M.: GEOTAR-Media, 2006.
2. Analysis of medicinal mixtures /A.P. Arzamastsev, V.M. Pechennikov, G.M. Rodionova et al. M.: Sputnik Company, 2000.
3. Belikov V.G. Pharmaceutical chemistry. In 2 hours: textbook, 4th ed., Revised. and additional Moscow: MEDpress-inform. 2007.
4. Guide to laboratory studies in pharmaceutical chemistry: E.N. Aksenova, O.P. Andrianova, A.P. Arzamastsev and others. M.: Medicine, 2001.
Hosted on Allbest.ru
...Similar Documents
Feedstock for the production of steroid hormones. Basic microbiological transformations of steroids. Hydrolysis of steroid esters, cleavage of side chains. Methods for conducting microbiological transformation processes, examples of their industrial use.
term paper, added 06/11/2014
Characteristics and classification of types of hormones. Characteristics of anabolic steroids. The mechanism of action of steroids. The effect of anabolic steroids on the body. Regulation of the activity of organs and tissues of a living organism. Peptide and protein hormones.
presentation, added 03/01/2013
The concept and functions of hormones. Microbiological transformations of steroids with industrial application. Raw material for the synthesis of steroid hormones. Genetic engineering method for obtaining somatostatin. Creation of insulin based on recombinant DNA technology.
presentation, added 12/22/2016
Definition and classification of anabolic steroids as chemicals that enhance protein synthesis. scope of their use. Useful effects of steroids. Causes of their adverse effects. Undesirable effect and consequences of long-term use.
abstract, added 11/26/2015
test, added 07.12.2010
The general concept of steroids - derivatives of a number of hydrocarbons, mainly pregnane, androstan, estran. Dosage forms of steroid drugs, their physical and chemical properties. The beginning of the use of glucocorticoids as drugs.
thesis, added 02/02/2016
Chemical nature of polypeptides, amino acids and their derivatives and fat-soluble steroids. The importance of the hypothalamus in providing communication between the nervous and endocrine systems. The role of the thyroid gland in the life of the body. The composition of the glands of mixed secretion.
presentation, added 03/24/2019
Hormones of the adrenal cortex. Scheme of the adrenal gland zones and the hormones they produce. The adrenal medulla. Side effects of glucocorticoid therapy. Disorders associated with the adrenal glands. Antihormonal drugs, indications for use.
lecture, added 04/28/2012
Structure, nomenclature and classification of steroid hormones, a review of their biosynthesis pathways. Enzymes involved in the biosynthesis of steroid hormones, their regulation. Mechanism of action, interaction with target cells. Features of inactivation and catabolism.
presentation, added 10/23/2016
The main tasks of toxicological chemistry. The role of chemical-toxicological analysis in the work of centers for the treatment of poisoning. Characteristics of the duties of an expert chemist. Influence of physical and chemical properties of poisons on their distribution and accumulation in the body.
DEPARTMENT OF THEORY AND METHODOLOGY OF GYMNASTICS
ABSTRACT
on the topic: “Chemistry of steroids. Their biological role.
Kovalenko
Vitaly Evgenievich
1st year student
group number 102
Malakhovka-2017
Introduction
1. Steroids and features of their structure
2. Steroids in action
3. The biological role of steroids
4. Pros and cons of steroids
Conclusion
Bibliography
Introduction
Steroids appeared more than 50 years ago, since the time when derivatives of the male sex hormone testosterone were created by artificial chemical means in the 40s. At first, steroids were used only for medical purposes, when additional stimulation of anabolic processes in human body. But soon these features of anabolic steroids were needed by professional athletes to achieve best results. So began to use anabolic steroids in big sport. At the moment, there are a number of steroids, comprising more than a hundred different names. All these steroids are derivatives of testosterone or substances close to it, and accordingly have its characteristic properties.
Steroids and features of their structure
Steroids are pharmacological preparations that, in their chemical structure and pharmacological action, are close to testosterone, being its derivatives. Steroids enhance the processes of synthesis of nucleic acids, as well as protein in cells, various enzymes, and due to this, they affect almost all types of metabolism in the body. As a result, anabolic steroids lead to an increase in body weight due to the growth of muscle tissue, a decrease in adipose tissue and an increase in the physical strength and endurance of the athlete's muscles. Steroids are distinguished by two effects of testosterone: androgenic and anabolic. The androgenic effect is manifested in the development of secondary male sexual characteristics, such as body structure, height, broad shoulders, narrow pelvis, masculine facial features, hair on the face and other parts of the body, deep voice, typical male sexual desire, aggressive behavior and much more. . The anabolic effect is mainly associated with accelerated muscle growth. Under the word steroids, one must mean, firstly, the desirability of anabolism, and secondly, as if in contrast to the term androgenic, they emphasize that steroids are made there in order to enhance the function of influencing muscle growth and inhibit the function of strengthening secondary sexual characteristics.
Steroids in action
The mechanism of action of steroids at the cellular level is very complex, however, at least a general, schematic representation of this process should be. Once in the blood, steroid molecules are carried throughout the body, where skeletal muscle cells, sebaceous glands, hair follicles, certain parts of the brain and some endocrine glands react to them. The selective accumulation of anabolic steroids and testosterone in the body is associated with the presence in the cells of the so-called "target organs" - specific molecular structures of a protein nature, which are called receptors. These intracellular receptors for testosterone, firstly, differ from the receptors of other steroids (estrogens, progestogens, corticosteroids, and others) and, secondly, interact with anabolic steroids as testosterone-related compounds. IN bound form the receptor-steroid complex is transported through the cytoplasm of the cell to the cell nucleus, where it interacts with proteins. As a result, the synthesis of all types of nucleic acids is stimulated and the process of formation of new protein molecules is “started”. These new molecules are either used inside the cell or released from the cells and carried by the blood (immunoglobulins, fibrinogen, blood transport proteins, and others). Another important area of anabolic action is the effect of anabolic steroids on the permeability and structure of cell membranes and subcellular components. As a result, nutrients, amino acids, vitamins, macro- and microelements, oxygen, glucose, fatty acids and many other molecules that are necessary for the physiological course of all metabolic processes actively enter cells and subcellular structures. Anabolic steroids stimulate the synthesis of creatine phosphate. It plays an important role in the restoration of adenosine triphosphate (ATP), which means the accumulation of energy in muscle cells. When needed, ATP passes into ADP (adenosine diphosphate) releasing a huge amount of energy, this explains the increase in strength, but not mass, for example, when using oxandrolone (synthesized a large number of CF in the muscle cell). The increase in endurance from the use of steroids is explained by the fact that in the muscle cell there is an increased supply of carbohydrates in the form of glycogen, except that goes still increased accumulation of fluid, as a result, muscle volume increases. ATP is directly involved in the functioning of contractile proteins muscle cells. Without it, there is no movement, speed and strength. In addition to creatine phosphate and ATP, there are other substances that perform energy functions, which are actively affected by anabolic steroids. These are glycogen and lipids (fats).
Thus, anabolic steroids stimulate the synthesis of new cells, boost energy production in the body.
The biological role of steroids
Consider the biological role of steroids on the example of cholesterol. Its biological role:
1. Component of cell membranes.
2. Needed for synthesis:
a) vitamin D;
b) bile acids;
c) hormones of the sex glands and the adrenal cortex.
3. Binding and transport of polyunsaturated fatty acids.
Cholesterol is present in all animal lipids, blood, bile. It is a source of formation in the body of bile acids, corticosteroids, sex hormones, vitamin D3, is a component of biological membranes. Approximately 20% of cholesterol enters the body with food. The main amount of cholesterol is synthesized in the body from acetic acid. Synthesis of cholesterol is carried out in the cells of almost all organs and tissues, however, cholesterol is formed in significant amounts in the liver (80%), the wall of the small intestine (10%) and skin (5%). Violation of cholesterol metabolism leads to deposition on the walls of blood vessels, as a result of which the elasticity of blood vessels decreases, atherosclerosis occurs, in addition, cholesterol can accumulate in the form of gallstones. However, there is not always a correlation between blood cholesterol levels and the severity of atherosclerosis. An increase in the concentration of cholesterol in the blood is observed in diabetes mellitus, hypothyroidism, gout, obesity, in some liver diseases, acute cerebrovascular accident. Reduced cholesterol is noted in a number of infectious diseases, intestinal diseases, hyperthyroidism.